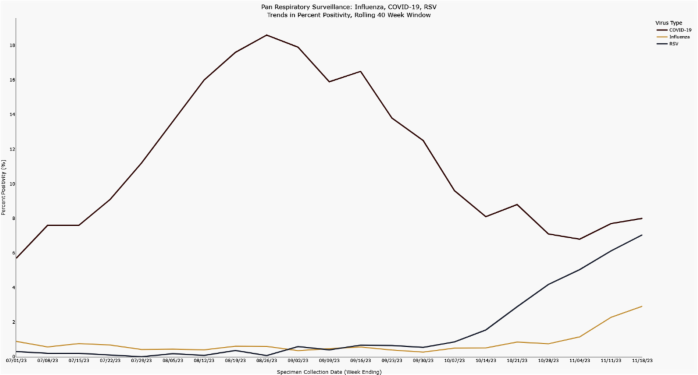
For the last several weeks there has been increasing respiratory virus activity in Orange County. Rates of COVID-19, Respiratory Syncytial Virus (RSV), and Influenza disease are all rising based on the percentage of positive test results reported to OCHCA. RSV activity has been increasing for the last 6 weeks, but more recently increases in influenza and COVID-19 activity have been detected. These patterns are in line with the regular seasonal epidemiology of these viruses.
Vaccines are now available for the prevention of disease caused by all three of these viruses. This season’s influenza and COVID-19 vaccines are recommended for all persons 6-month of age and older, while RSV vaccines or nirsevimab monoclonal antibody are recommended for select populations. Based on data reported to the California Immunization Registry (CAIR), ~740,000 influenza vaccines have been administered in Orange County through week 46, while only ~260,000 doses of the current season’s COVID-19 vaccines and ~ 52,000 doses of RSV vaccine have been administered.
Prevention
- Recommend patients use nonpharmaceutical interventions (NPIs), including staying home when sick and practicing consistent cough and hand hygiene and masking to decrease their risk of contracting and spreading respiratory viruses.
- Recommend influenza and COVID-19 vaccination to all patients 6-months and older. Current recommendations for influenza vaccination, and COVID-19 vaccination are linked.
- Recommend RSV prevention for appropriate groups as follows:
- RSV vaccination, using shared decision making for adults > 60 years of age.
- Pfizer’s RSV vaccine is recommended for pregnant women 32-36 weeks gestation seasonally, between September and January, to prevent RSV disease in their newborns.
- Nirsevimab, a long-acting anti-RSV monoclonal antibody is recommended for use in infants and young children to prevent severe RSV disease. Unfortunately, there are limited supplies of nirsevimab available this season. A recent CDC Health Advisory on the limited supply of nirsevimab provided guidance to providers on making use of available product.
- COVID-19, influenza and RSV vaccines may be administered at the same visit for those eligible for all three.
Treatment
- Providers should continue to consider treatment for COVID-19 disease particularly those at risk for severe disease. The latest recommendations for hospitalized and non-hospitalized children and adults available with the NIH COVID-19 Treatment Guidelines.
- Providers should consider influenza antiviral medications for both treatment and for chemoprophylaxis of influenza. Recommendation for use during the 2023-2024 season can be found at CDC’s Influenza Antiviral Medications: Summary for Clinicians.